Chemistry I
Chapter 2: Atoms, Molecules, and Ions
Atomic Structure
- Protons (positively charged); in nucleus
- Neutrons (neutral); in nucleus
- Electrons (negatively charged); electron cloud surrounding nucleus
Neutral atom have equal number of protons and electrons; ions have unequal amounts of protons and electrons.
- Cations have more protons than electrons
- Anions have less protons than electrons
Discovery of the structure of the atom
Source: Wikimedia
Atomic Symbolism
Element Symbol (X) – the symbol of the element
Atomic Number (Z) – number of protons; defines the element
Mass Number (A) – number of protons + neutrons
\[\Huge{^{\mathrm{A}}_{\mathrm{Z}}\mathrm{X}}\]
Atomic Symbolism
Helium-4 represented below. Atomic number is often omitted (middle). Charge can be included (right).
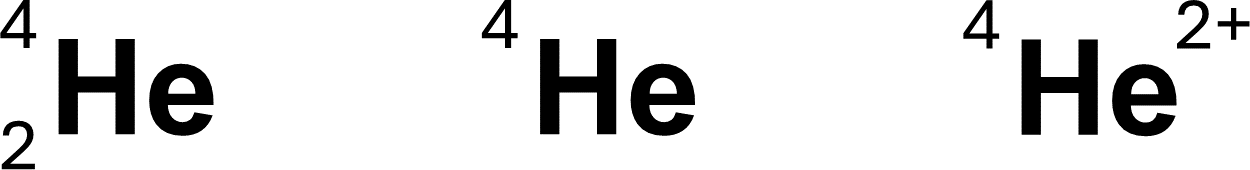

Subatomic Particles
The Dalton (Da), or unified atomic mass unit (u), is a unit of mass that describes the mass of an individual particle. It is approximately equal to the atomic mass unit (amu), an outdated but commonly used unit of mass.
\[1~\mathrm{Da} = 1~\mathrm{u} = 1.66053906892\times 10^{-27}~\mathrm{kg}\]
This conversion is inexact.
Isotopes
Isotopes are elements that have the same atomic number but differing mass numbers as a result of a varying number of neutrons.
Some elements are monoisotopic. See Wikipedia and IUPAC. Most elements have more than one isotope.
Hydrogen exists in three naturally occurring isotopes in different percent abundances.
| Name | Isotope | % Abundance | Abundance | Abundance (x:H ratio) | half-life (t½) |
|---|---|---|---|---|---|
Protium |
1H |
99.9855 % |
0.999855 |
1:1 |
stable |
Deuterium |
2H |
0.0145 % |
0.00145 |
1:6,900 |
stable |
Tritium |
3H |
trace |
trace |
1:1017 |
12.32 y |
See trace. Ratio determined by 1/(percent abundance)×100.
Dalton’s Modern Atomic Theory
John Dalton
- Matter is made up of atoms
- Atoms are indivisible and indestructible
- Atoms of an element type are identical
- Atoms of different elements have differing weights and chemical properties
- Atoms remain unchanged even if a compound decomposes

Source: Wikimedia
Discovery of the Electron
J.J. Thomson (1897) and the Cathode Ray Tube Experiment
- Determined the charge-to-mass ratio of the electron (1.7588 × 1011 C kg–1) by deflecting cathode rays (made of particles)
- This ratio was the same regardless of the cathode material which indicated that electrons are a fundamental component of all atoms
- Realized electrons were 3 orders of magnitude smaller than hydrogen atom
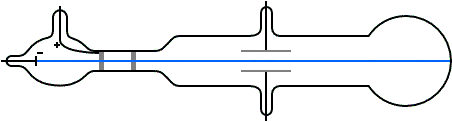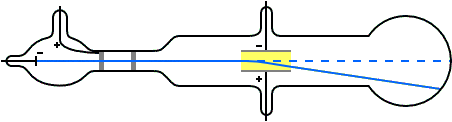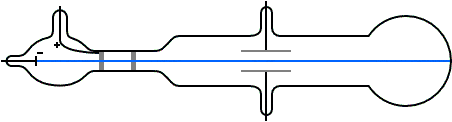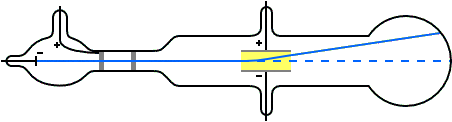

J. J. Thomson M.A.F.R.S. (1897): XL. Cathode Rays, Philosophical Magazine Series 5, 44:269, 293-316 doi: 10.1080/14786449708621070
As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter. The question next arises, What are these particles ? are they atoms, or molecules, or matter in a still finer state of subdivision?

Plum-Pudding Model
J.J. Thomson scientific model of the atom (1897)
- Atom is a collection of positive and negative charge
- Electrons spread throughout structure
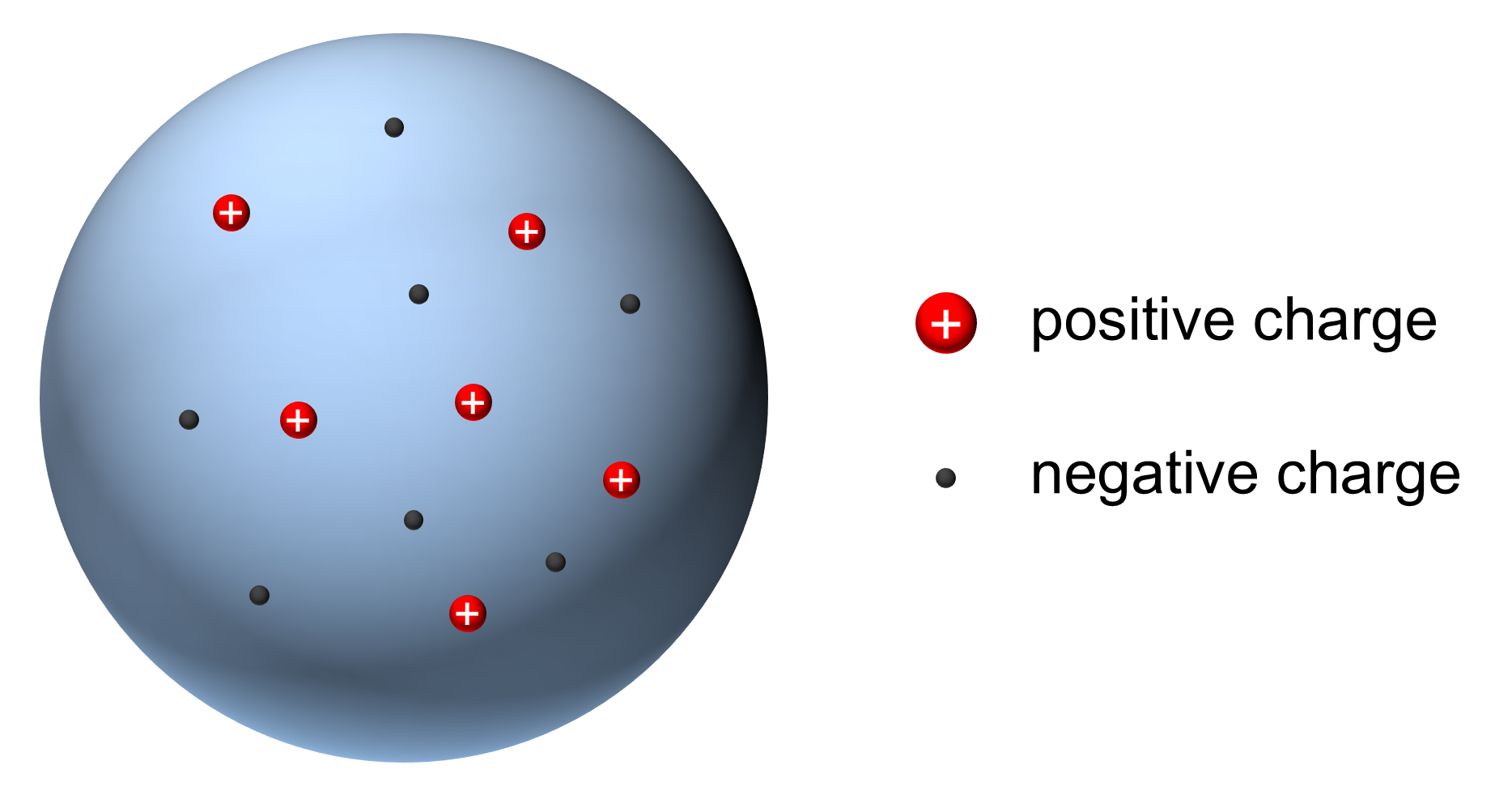
Discovery of Electron Charge
Robert Millikan (1908) and the Oil Drop Experiment
Oil droplets’ rate of fall tuned with an electric field
After many experiments, determined the charge of an electron was a small integer multiple of a certain base value.
Proposed: 1.524(17)×10–19 C (0.6 % error)
Actual: 1.602 176 634× 10–19C (exact)
Source: Wikimedia
Controversy
- Millikan did not include all the data from his experiments in his reports.
- The omitted data were “outliers” from what Millikan published
- The uncertainty would have been larger if all data had been included
Would discovery for the actual electron charge been faster had the data not been doctored?
Psychological effects in scientific methodology (Wikipedia)
We have learned a lot from experience about how to handle some of the ways we fool ourselves. One example: Millikan measured the charge on an electron by an experiment with falling oil drops, and got an answer which we now know not to be quite right. It’s a little bit off because he had the incorrect value for the viscosity of air. It’s interesting to look at the history of measurements of the charge of an electron, after Millikan. If you plot them as a function of time, you find that one is a little bit bigger than Millikan’s, and the next one’s a little bit bigger than that, and the next one’s a little bit bigger than that, until finally they settle down to a number which is higher.
Why didn’t they discover the new number was higher right away? It’s a thing that scientists are ashamed of—this history—because it’s apparent that people did things like this: When they got a number that was too high above Millikan’s, they thought something must be wrong—and they would look for and find a reason why something might be wrong. When they got a number close to Millikan’s value they didn’t look so hard. And so they eliminated the numbers that were too far off, and did other things like that…
Richard Feynmann (1974)
Discovery of the Nucleus
Ernest Rutherford (1910) and the Gold Foil Experiment
- Shot α particles (helium nucleus) at thin gold foil
- Expected to fly through unperturbed
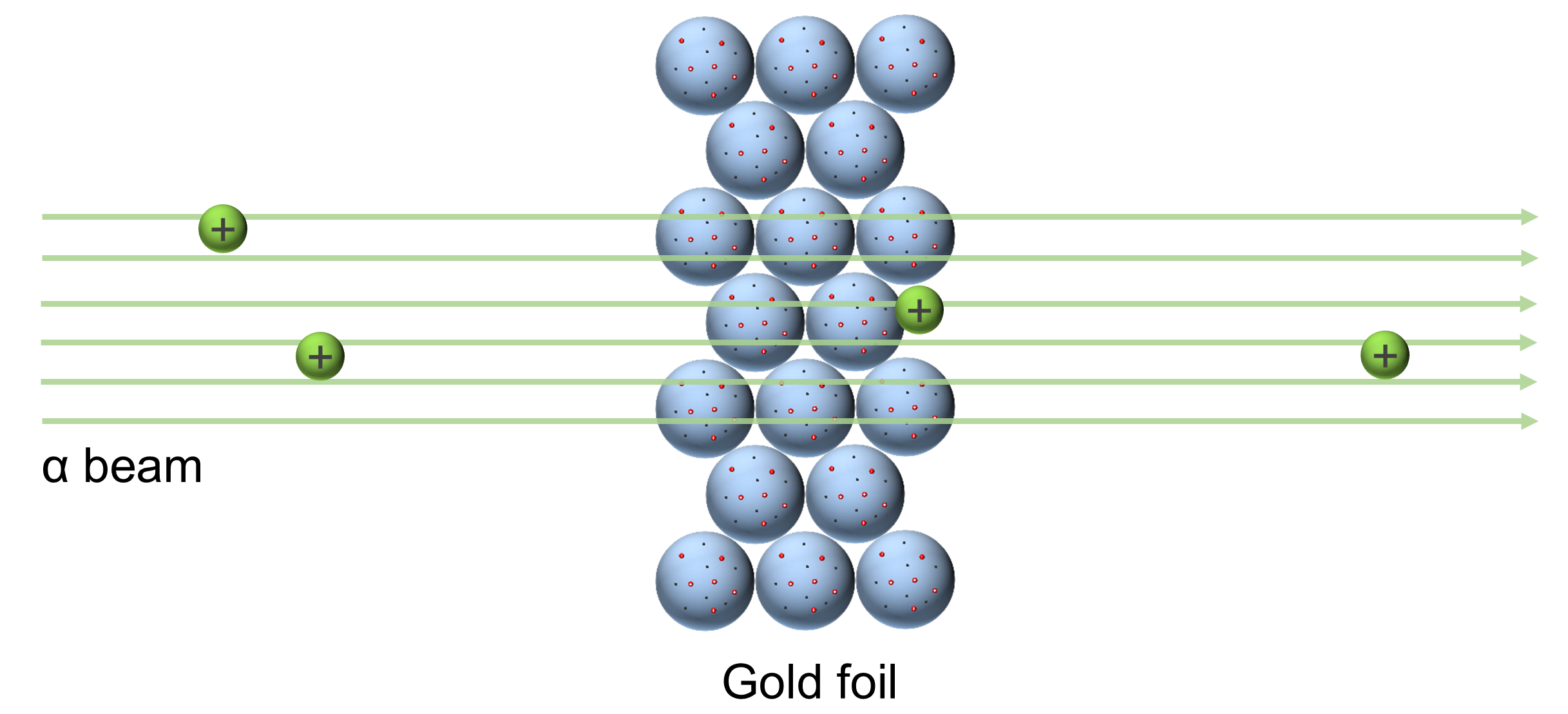
Result: Some particles were deflected; some came back toward the detector
Conclusion: Scattering was caused by a hard, dense core at the center of the atom (now called the nucleus)
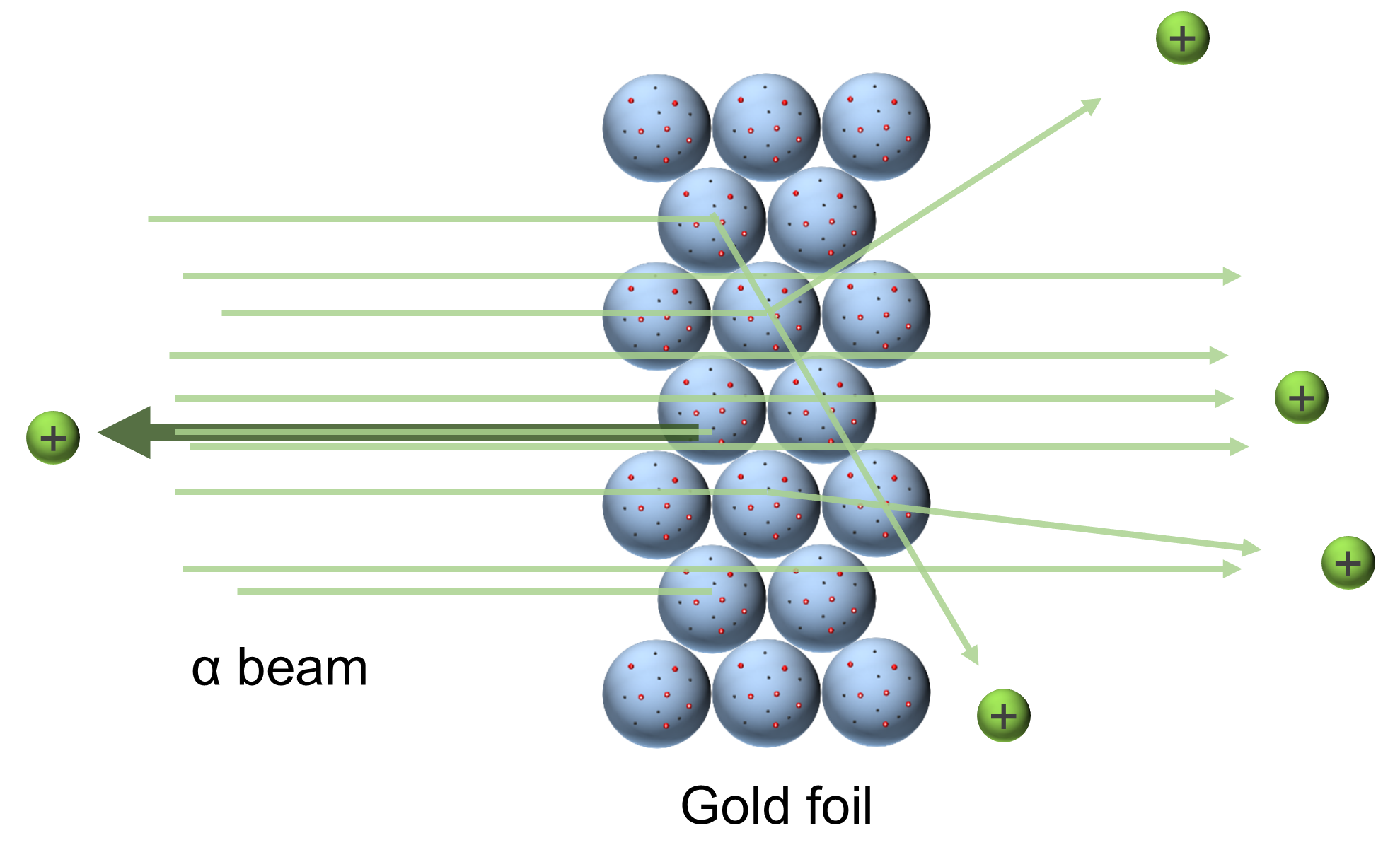
Source: Wikimedia
He could hardly believe what he saw. He tested and retested every aspect of the experiment, but when he couldn’t find anything wrong, he reported the results to Rutherford.
Rutherford too was astonished. As he was fond of saying, “It was as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Rutherford determined the nucleus to be 1/100,000 the size of the atom. The atom was mostly empty space.
If a basketball were a nucleus, the atom would be “city sized”.
Quote source: APS News
The Periodic Table
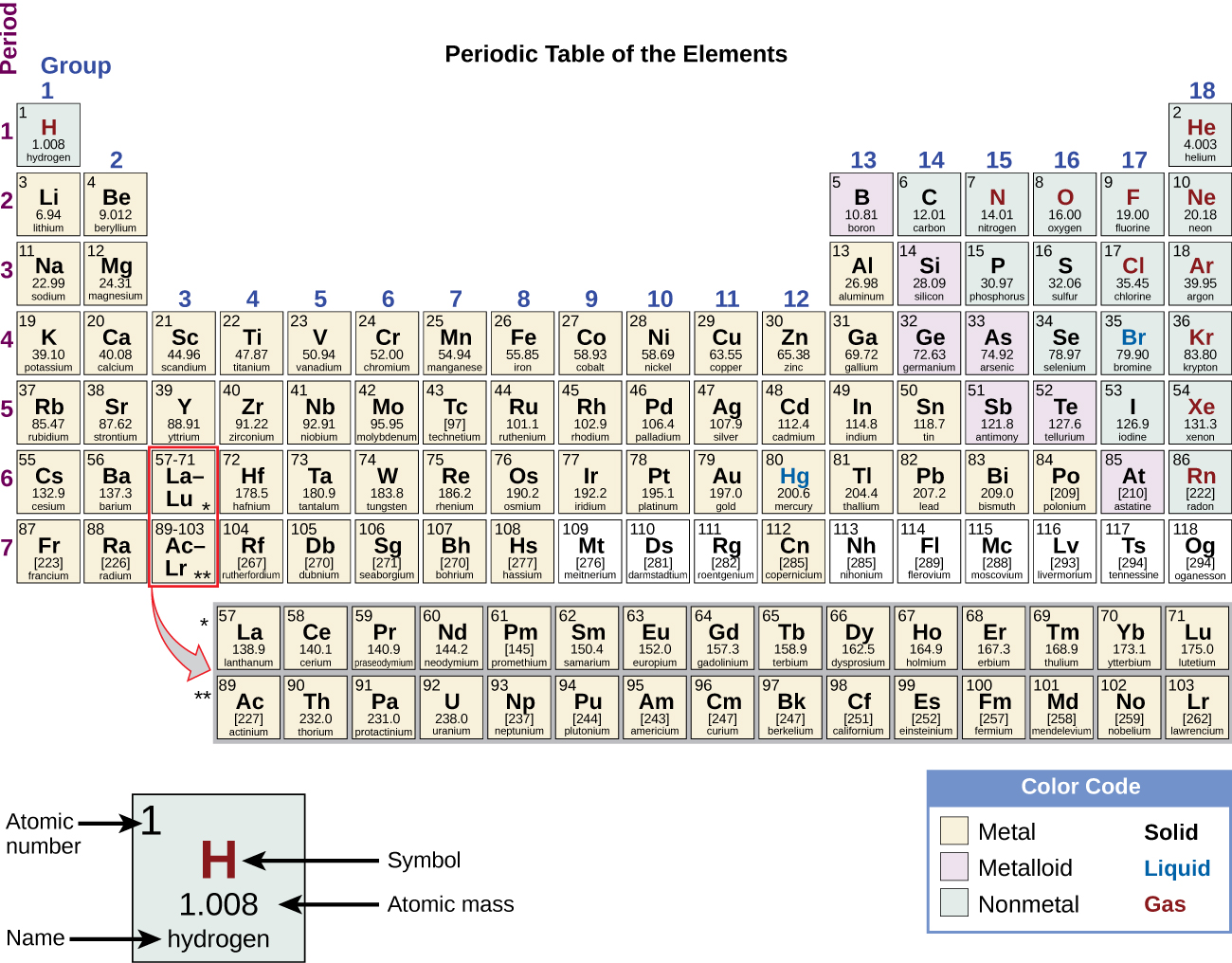
Source: OpenStax
Group - columns
Period - rows
Regions - metals, metalloids, nonmetal
Group 1 - alkali metals
Group 2 - alkaline earth metals
Group 15 - pinctogens
Group 16 - chalcogens
Group 17 - halogens
Group 18 - noble gases
Elements to Know
- First 5 periods
- Other elements you see used (e.g. in examples, practice, homework) like Cs, W, Os, Ir, Pt, Au, Hg, Bi, Po, U, etc. (list not complete)
The masses given on the periodic table are standard atomic weights, or more precisely, abridged standard atomic weights. Fourteen elements (H, Li, B, C, N, O, Mg, Si, S, Cl, Ar, Br, Th, and Pb) have standard atomic weights that are defined by an interval, but their abridged atomic weights are given on a typical periodic table.
The mass of a singular atom/isotope is the atomic mass.
Ions
For any neutral element
- add electron → decrease charge
- remove electron → increase charge
\[ \huge{\mathrm{F} + e^- \longrightarrow \mathrm{F^-}}\\[6ex] \huge{\mathrm{Mg} \longrightarrow \mathrm{Mg^{2+}}+ 2e^-} \]
Ionic bonds
Neutral ionic compounds can form compounds through the combination of cations and anions.
\[\huge{\mathrm{Ca^{2+}} + \mathrm{CO_3^{2-}} \longrightarrow \mathrm{CaCO_3}}\]
\[\huge{\mathrm{Ca^+} + \mathrm{2~\!Cl^-} \longrightarrow \mathrm{CaCl_2}}\]
Nomenclature
See Nomenclature
